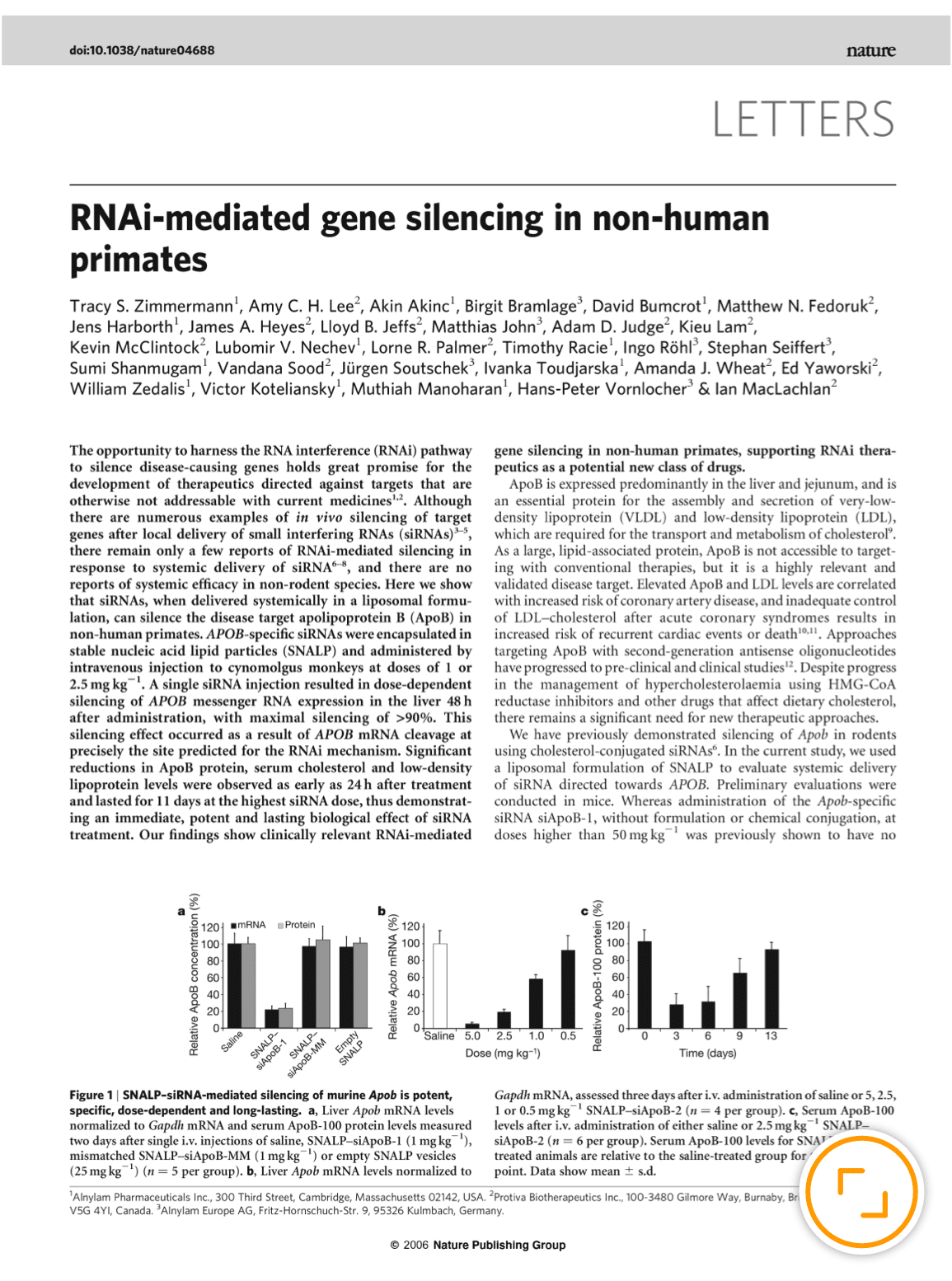
Celebrating 20 Years
of Alnylam
-
Innovation
-
Leadership
-
Perseverance
-
Innovation
And What’s Next in the

Scroll to Start Your Experience

Revolutions are born from the aspirations of people who believe there’s a better way.
Harnessing a breakthrough discovery in biology to treat disease in a better way, and transform the lives of patients in doing so, has been the mission for Alnylam from day one. Our founders believed that a new approach, leveraging small interfering RNA (siRNA) and the body's natural process for gene regulation - called RNA interference (RNAi), could be used to treat patients in ways that other classes of medicines - such as small molecules and antibodies - could not. They also believed that the unique characteristics of RNAi opened up a broader range of disease targets, including many which had been deemed “undruggable.”



Alnylam cofounder and Nobel Laureate Dr. Phillip Sharp first published a paper on using RNA interference and double-stranded RNA to silence genes in mammalian cells in Genes & Development in 1999. This paper would prove to be the catalyst for the collaboration between Alnylam’s five founders that would ultimately lead to the creation of the company in 2002.
READ THE PAPER › READ THE PAPER ›








Alnylam has led the RNAi Revolution®
Performing the earliest and foundational research into how to utilize RNAi to silence genes that cause disease, Alnylam pioneered the first phase of the RNAi Revolution. Through sustained research and development efforts over more than 15 years, our work yielded the critical breakthroughs and innovations that made the field of RNAi therapeutics possible.
Phillip Sharp, David Bartel, Thomas Tuschl and Phillip Zamore conducted the first experiments to try to develop the biochemistry of RNAi in vitro. Having confirmed the ability to go from double-stranded RNA to silencing of messenger RNA (mRNA) in a test tube, they moved on to other critical research that gave them the confidence to found Alnylam with Paul Schimmel (not pictured).
Hear Phil Sharp tell the story of how Alnylam’s founders came together to work on RNAi.

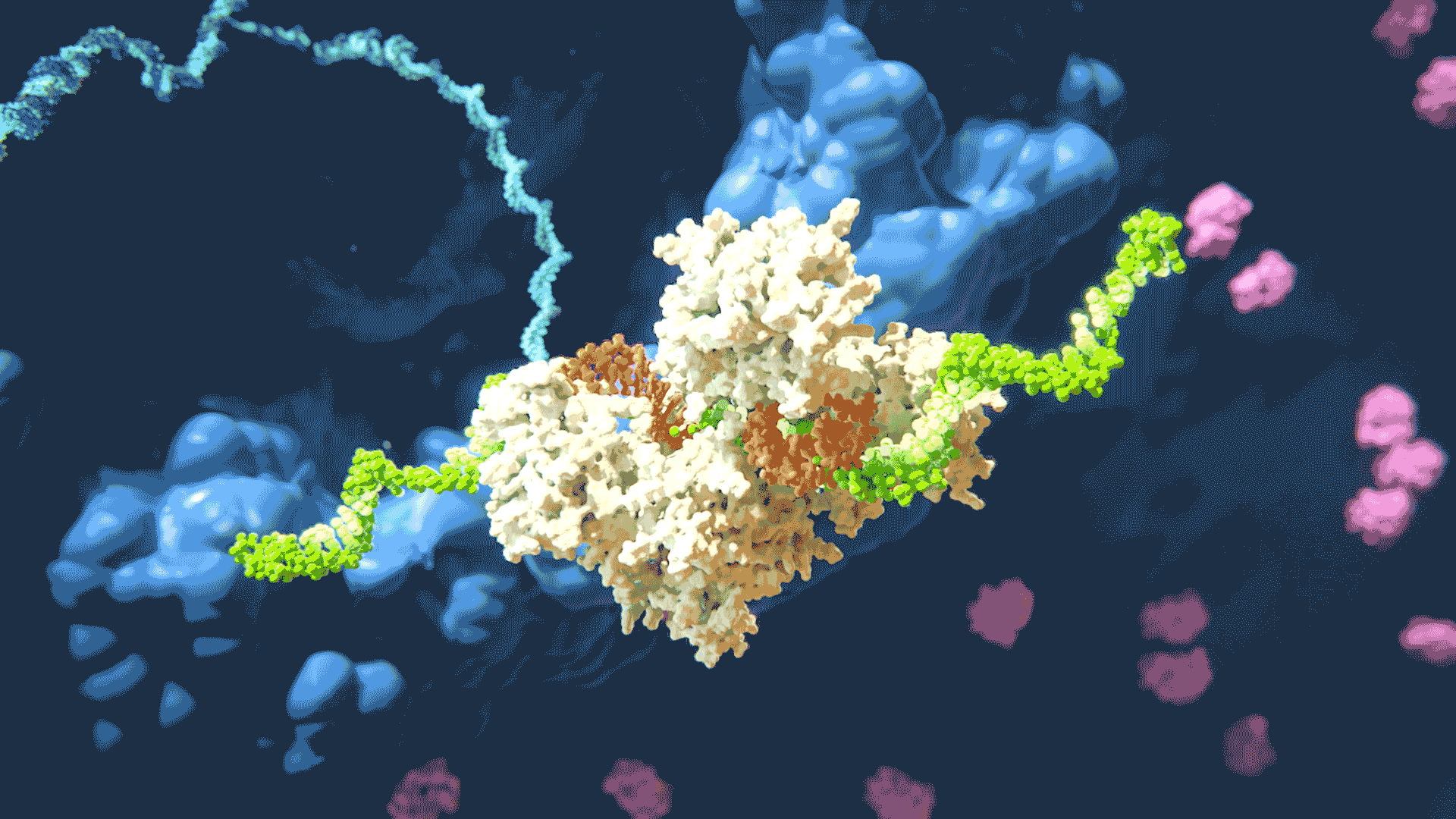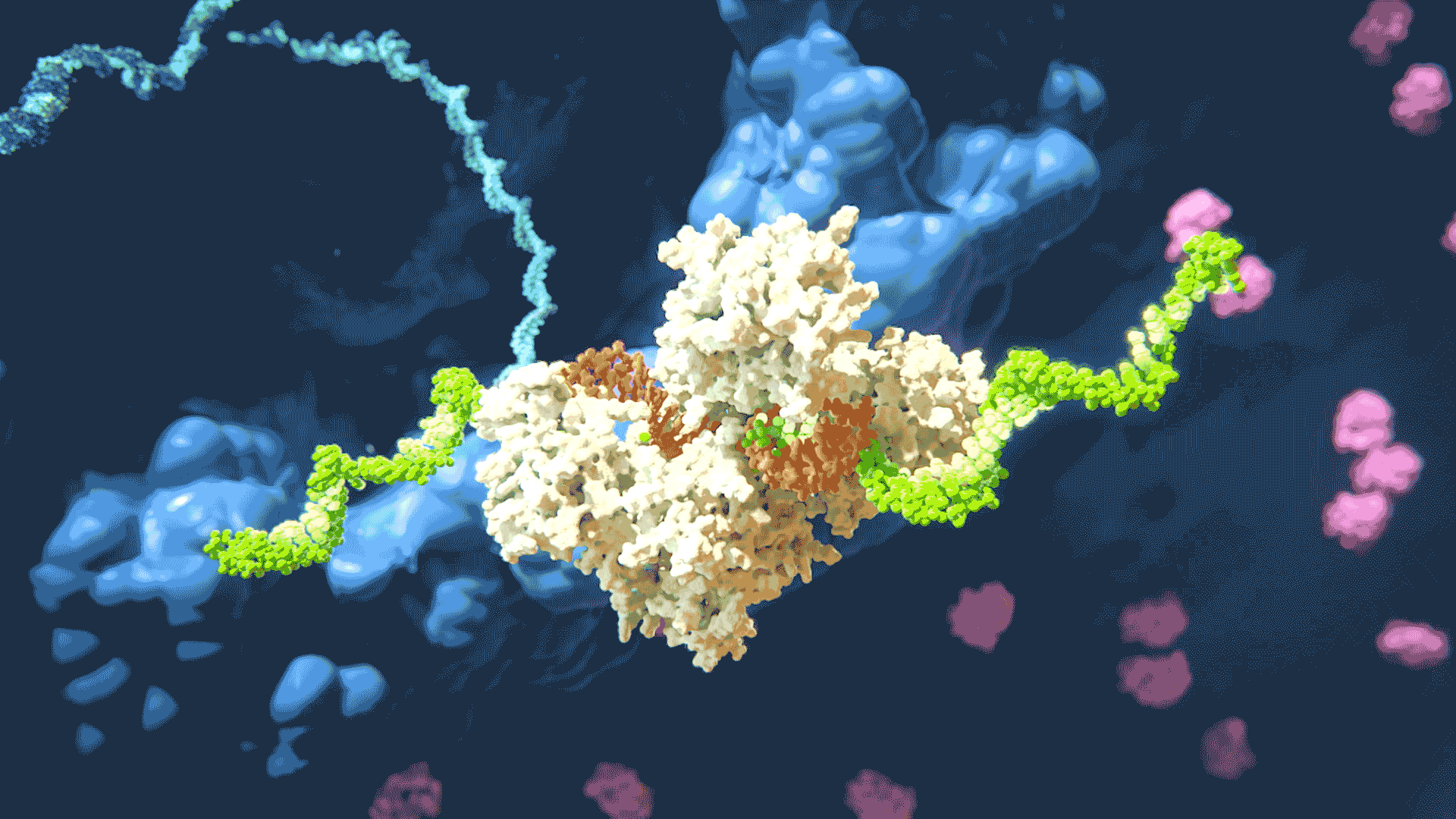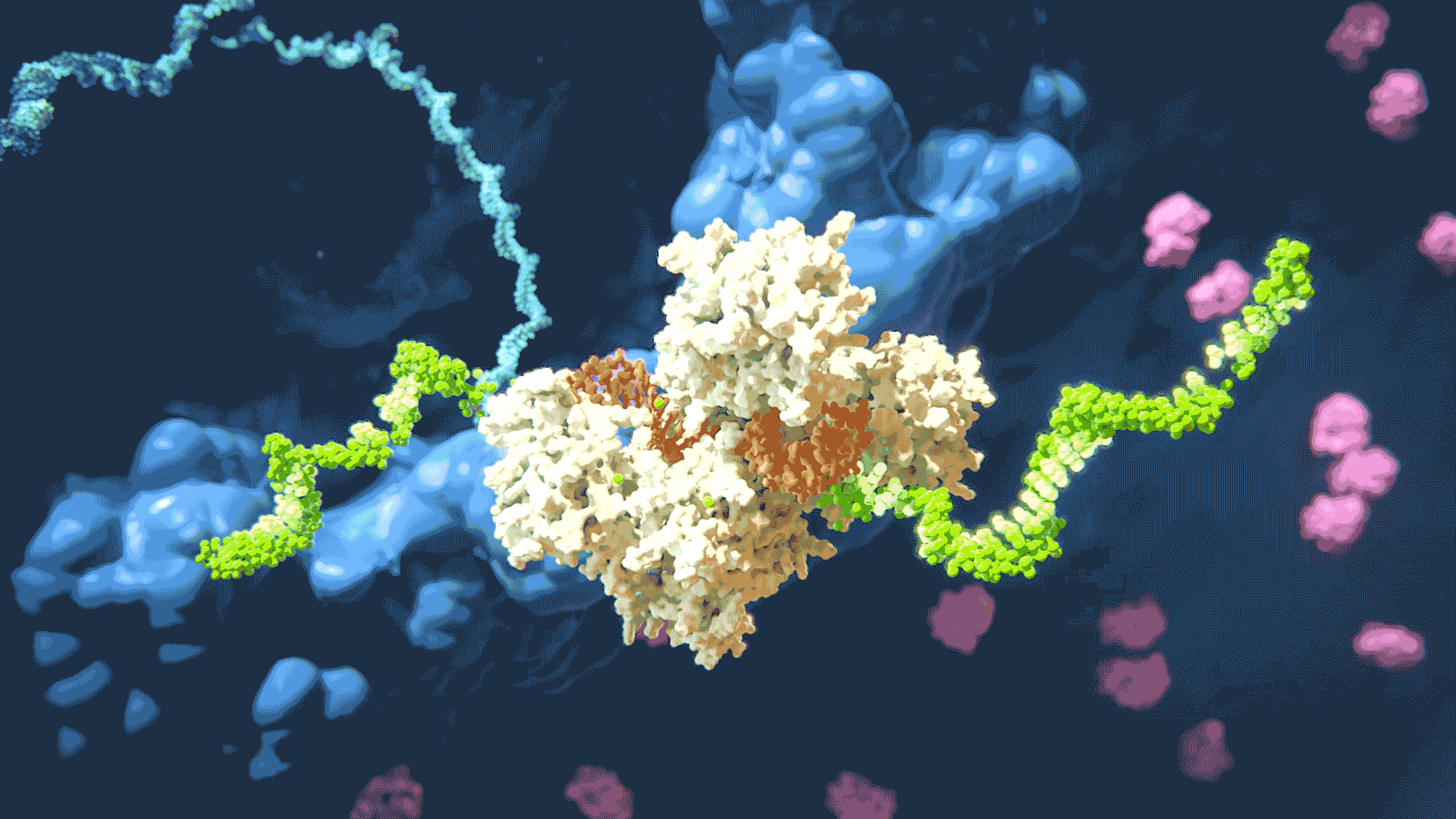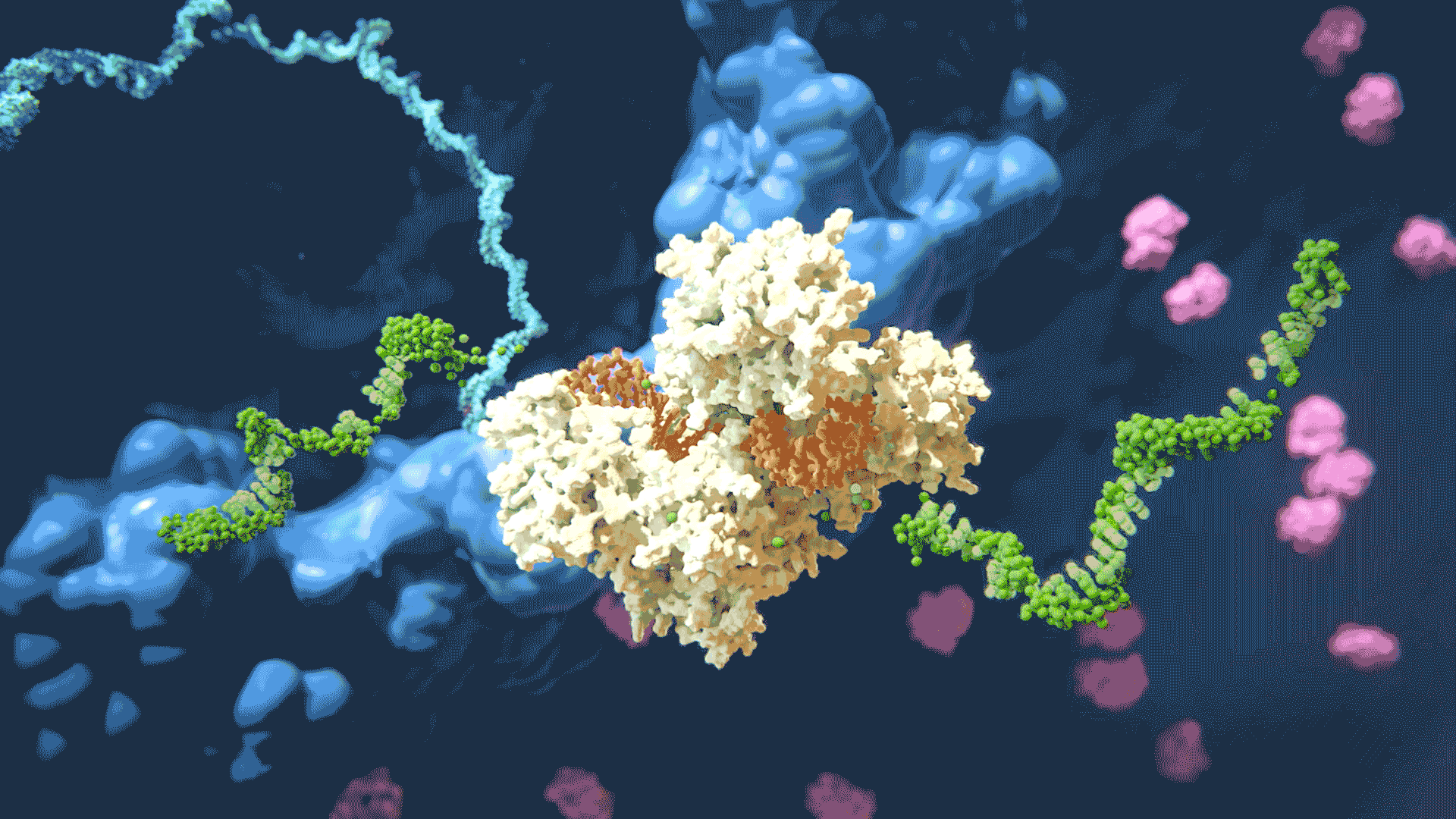
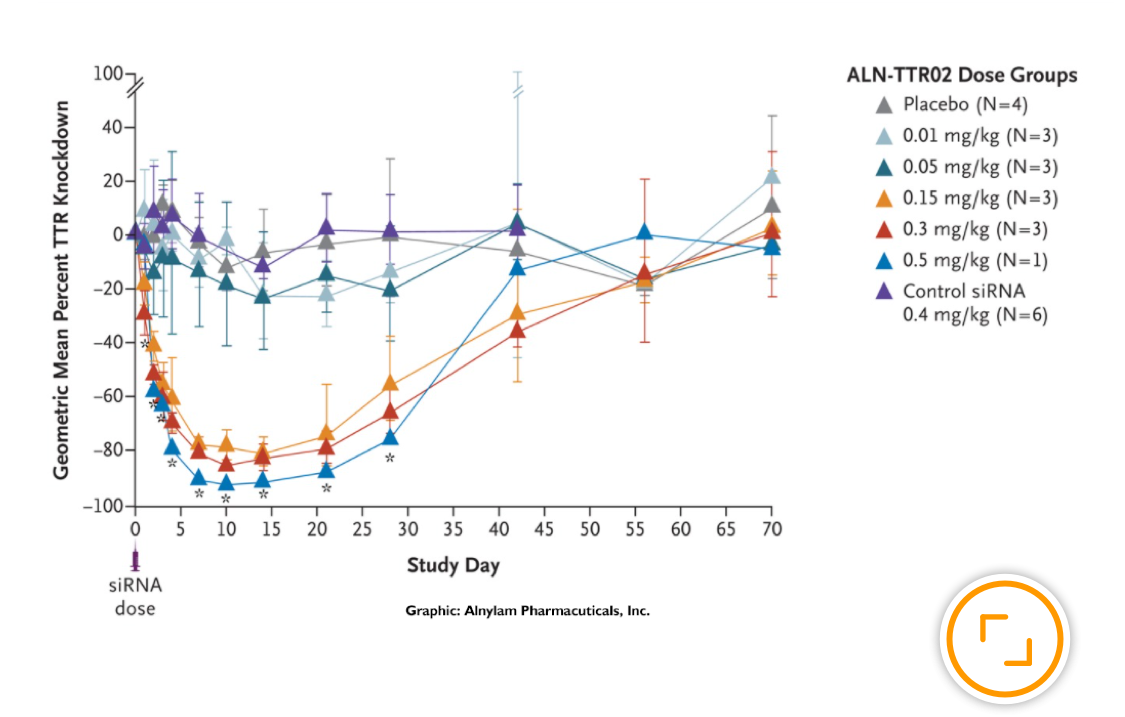
The RNAi process “silencing” gene expression by disabling target mRNA



Rare Diseases Prove the Concept, and Prove to be Just the Beginning
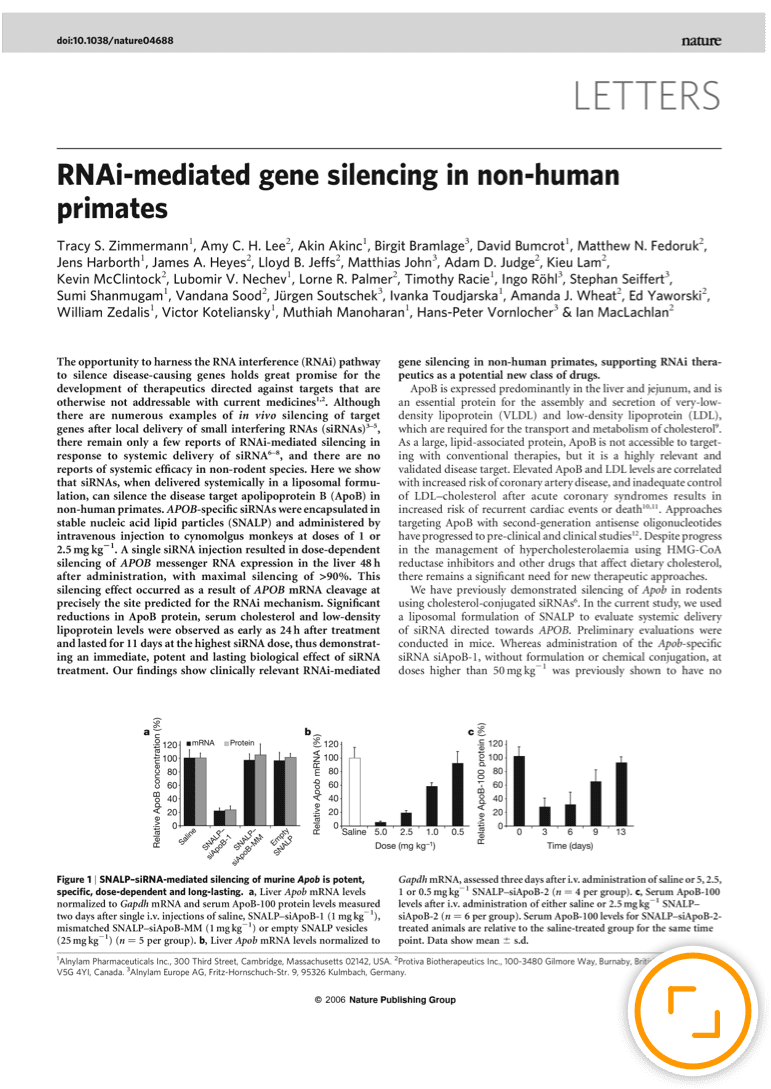
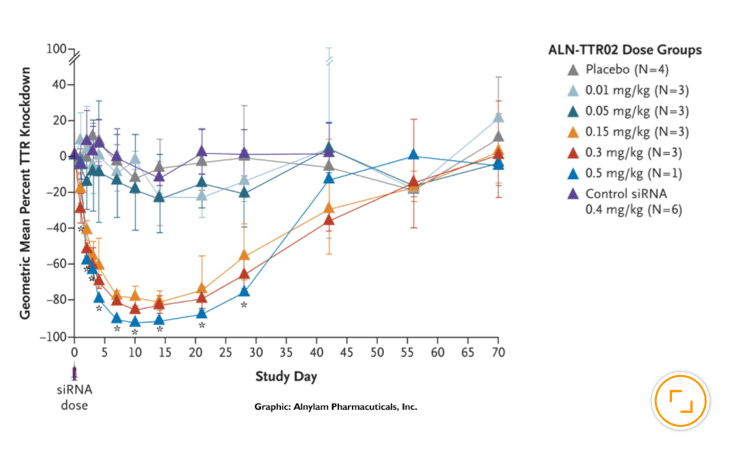
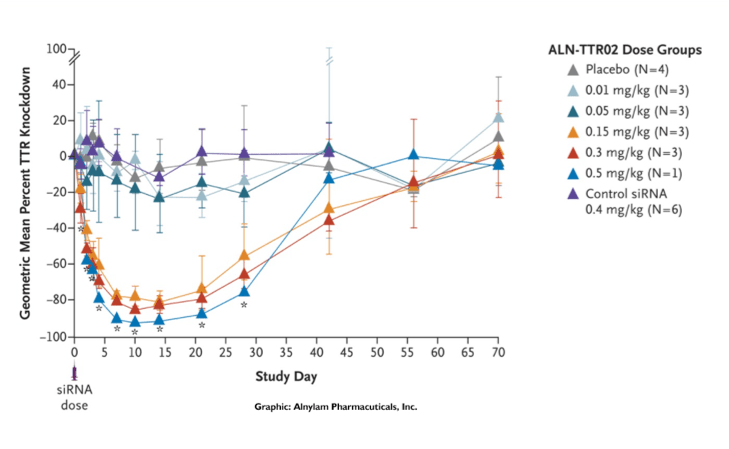
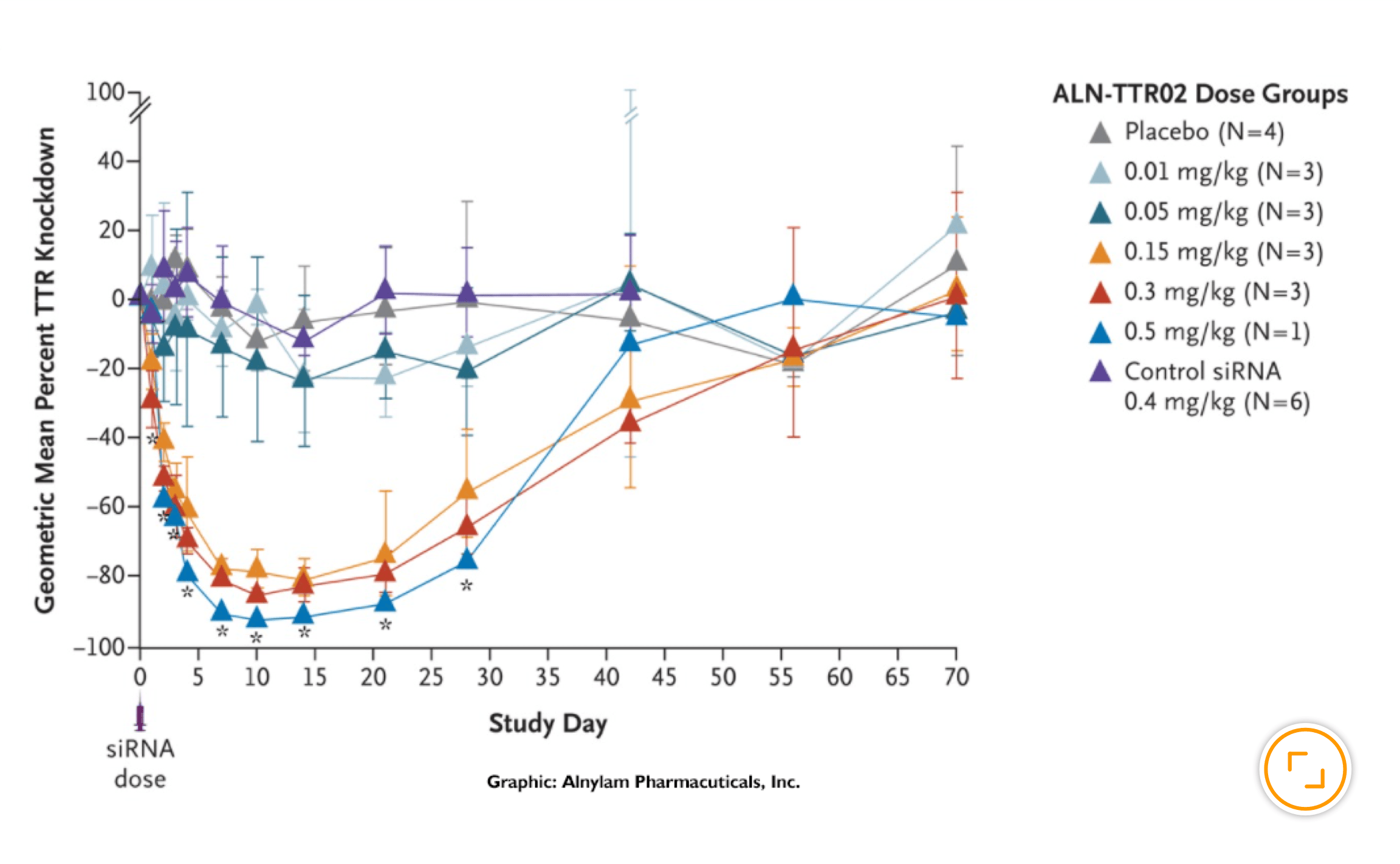
Alnylam’s ingenuity and perseverance in solving the challenges presented by pioneering science established a revolutionary new approach to drug development that enables rapid advancement of new therapies for patients who are waiting. People impacted by rare and genetic diseases, who had no, or very limited treatment options, were the first to benefit from this new class of medicines when the world’s first four (and only) RNAi therapeutics were approved in quick succession. But broader application of the RNAi approach was already well underway. Today, our robust pipeline includes several investigational medicines in diseases including hypertension, hemophilia, hepatitis B and Alzheimer's disease.
The Wall Street Journal article
Published: August 10, 2018